Biscoff - Lotus - 250 g
This product page is not complete. You can help to complete it by editing it and adding more data from the photos we have, or by taking more photos using the app for Android or iPhone/iPad. Thank you!
×
Côd bar: 5410126716016 (EAN / EAN-13)
Common name: The original caramelised biscuit
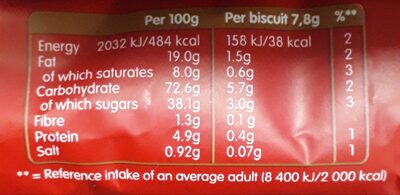
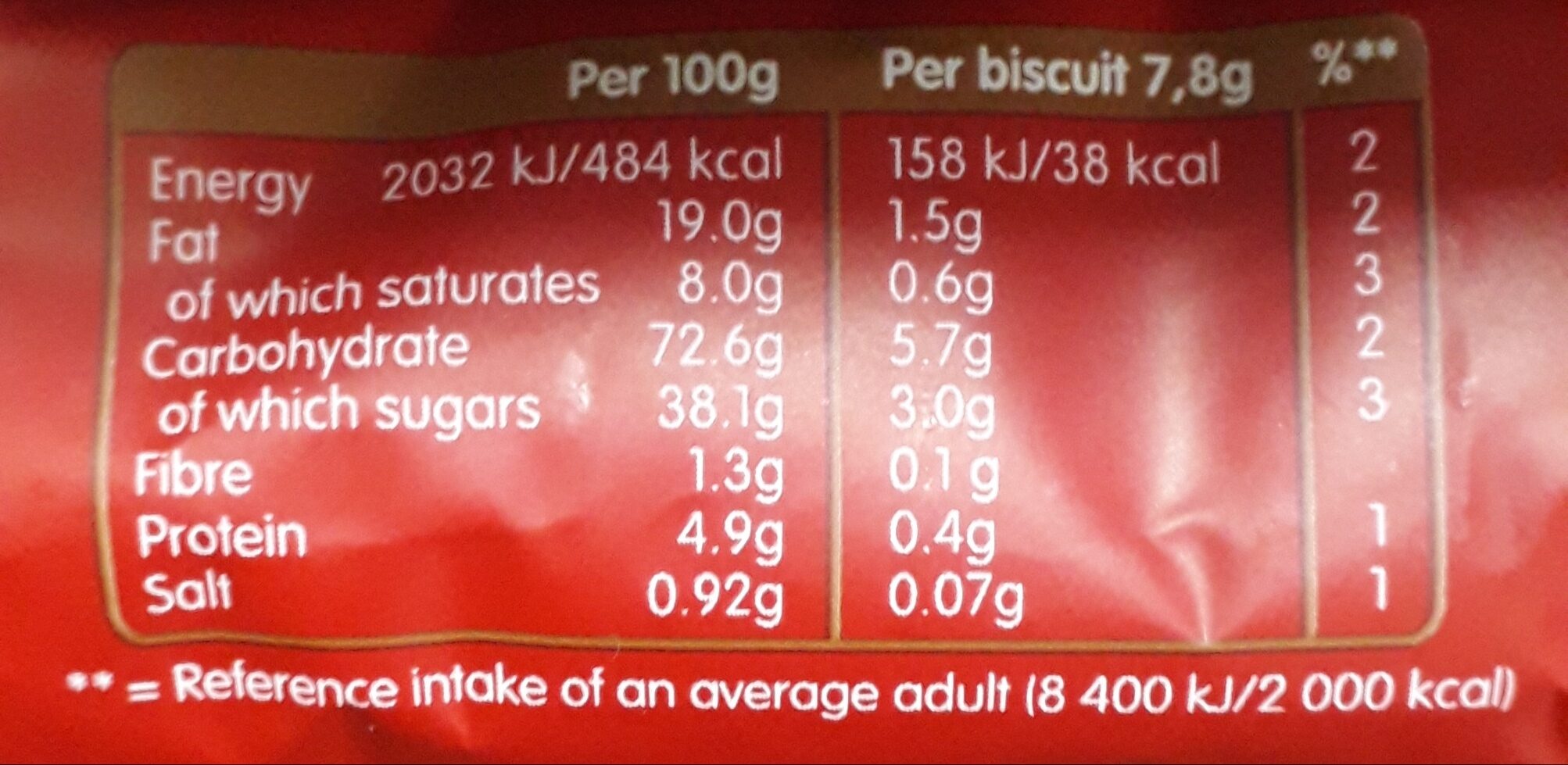
Quantity: 250 g
Packaging: en:Plastic, en:Mixed plastic-packet
Brandiau: Lotus
Categorïau: en:Snacks, en:Sweet snacks, en:Biscuits and cakes, Bisged
Labels, certifications, awards: en:Sustainable, en:Vegetarian, en:No artificial flavors, en:Sustainable Palm Oil, en:Vegan, en:100% natural, en:Contains palm oil, en:No colorings
Manufacturing or processing places: Belgium
Link to the product page on the official site of the producer: http://lotusbiscoff.com
Stores: El Corte Inglés, Hipercor, Supersol, Eroski, Caprabo, Condis, Amazon, Carrefour, Yerevan City, Dunnesstores, Tesco, ՍԱՍ, Froiz, Sainsbury's
Countries where sold: Armenia, Gweriniaeth Iwerddon, Seland Newydd, Portiwgal, Y Deyrnas Unedig
Matching with your preferences
Environment
Carbon footprint
Packaging
Transportation
Threatened species
Report a problem
Data sources
Product added on gan kiliweb
Last edit of product page on gan lcmortensen.
Golygwyd y tudalen cynnyrch hefyd gan alexg, alia, beniben, caylingo, ecismygame, ecoscore-impact-estimator, federfico, foodrepo, guezguez-majed, inf, insectproductadd, lee-carre, martinshadok, musarana, neptuno, o-andras, openfoodfacts-contributors, packbot, planteuser, prepperapp, roboto-app, scanbot, swipe-studio, thaialagata, vikalex, vishaldh, yousshap, yuka.SC8wQVFJOEdwcVJieE1ZTzB3clVwc3BNemFPVFFrYTFMZEFNSWc9PQ, yuka.UUk5YU5Zc1FuT2RidzhZKzR4UHIyK05NMXJ5NVdUNkdjZkJLSVE9PQ, yuka.WW80K01Qc3FxS2NxbS9adXBRL0lwUFYvMktHQVJVNlRkK2NXSWc9PQ.